Potassium bicarbonate, a versatile chemical compound, finds applications across various industries, from agriculture to food processing and pharmaceuticals. Its role as a buffering agent, leavening agent, and pH balancer underscores its importance. Understanding the production process of potassium bicarbonate is crucial for stakeholders in the industry, as it provides insights into cost structures, raw material requirements, and potential market dynamics. This blog delves into the comprehensive production process, associated costs, and the latest developments in the potassium bicarbonate market.
Potassium Bicarbonate Production Cost
The production cost of potassium bicarbonate is influenced by several factors, including raw material costs, energy consumption, labor, and overhead expenses. The process involves the reaction of potassium carbonate with carbon dioxide and water, which necessitates a reliable supply of these raw materials. Additionally, maintaining efficient production facilities to minimize energy consumption and optimize labor use is crucial. The overall cost structure can be broken down into fixed and variable costs, with raw materials often constituting the largest portion.
Request For Sample: https://www.procurementresource.com/production-cost-report-store/potassium-bicarbonate/request-sample
Manufacturing Report and Process
Step-by-Step Production Process
- Preparation of Potassium Carbonate Solution: The production of potassium bicarbonate begins with dissolving potassium carbonate (K2CO3) in water. This forms a potassium carbonate solution, which serves as the primary reactant.
- Carbon Dioxide Introduction: Carbon dioxide (CO2) is then bubbled through the potassium carbonate solution. This step is critical as it leads to the formation of potassium bicarbonate through a chemical reaction:
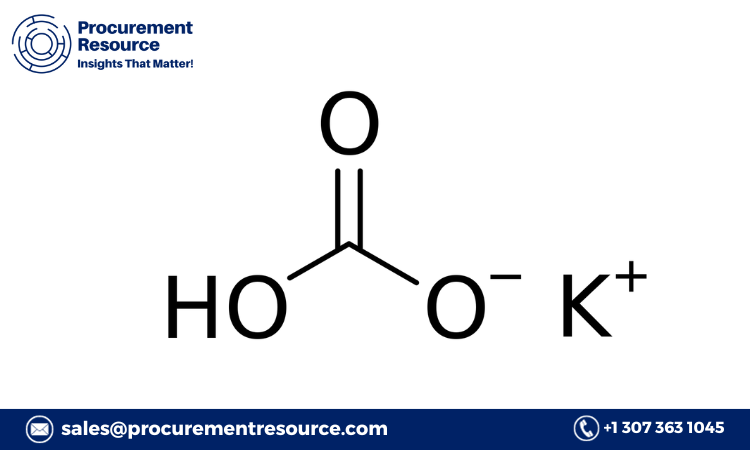
K2CO3+CO2+H2O→2KHCO3K2CO3 + CO2 + H2O \rightarrow 2 KHCO3
- Crystallization: As the reaction proceeds, potassium bicarbonate (KHCO3) starts to crystallize out of the solution. The conditions, such as temperature and concentration, are carefully controlled to maximize yield and ensure the formation of high-purity crystals.
- Filtration and Drying: The crystallized potassium bicarbonate is separated from the solution through filtration. The collected crystals are then washed to remove any impurities and subsequently dried to achieve the desired moisture content.
- Packaging: Finally, the dried potassium bicarbonate is packaged in suitable containers for storage and distribution. Packaging is done under controlled conditions to prevent contamination and moisture absorption.
Raw Material Costs
The primary raw materials required for the production of potassium bicarbonate are potassium carbonate, carbon dioxide, and water. Each of these materials contributes to the overall production cost.
- Potassium Carbonate: This is the most significant cost component. The price of potassium carbonate can fluctuate based on market demand, availability, and production costs of its own raw materials.
- Carbon Dioxide: CO2 is generally sourced as a by-product from industrial processes such as ammonia production or fermentation. The cost of CO2 can vary depending on the source and regional availability.
- Water: While water is a relatively inexpensive raw material, the cost associated with its purification and the maintenance of a consistent supply can add to the production expenses.
Latest News
The production and application of potassium bicarbonate have seen several notable developments in recent times:
- Sustainable Production Methods: With the increasing emphasis on sustainability, research is being conducted to develop more eco-friendly production processes for potassium bicarbonate. Innovations such as utilizing renewable energy sources for production and capturing CO2 from industrial emissions are being explored to reduce the environmental footprint.
- Market Growth: The market for potassium bicarbonate is expected to grow significantly in the coming years, driven by its expanding use in agriculture as a fertilizer and soil amendment, as well as in the food industry as a leavening agent. The rising demand for organic and environmentally safe products is also contributing to this growth.
- Technological Advancements: Advancements in technology are leading to more efficient production processes. Automation and improved process controls are enhancing production efficiency, reducing waste, and ensuring higher product quality.
- Regulatory Developments: Regulatory bodies are increasingly focusing on the safety and environmental impact of chemical production processes. Compliance with stringent regulations is driving manufacturers to adopt best practices and innovate in their production methods.
- Global Supply Chain Dynamics: The global supply chain for raw materials such as potassium carbonate and carbon dioxide is witnessing changes due to geopolitical factors and trade policies. Manufacturers are adapting by diversifying their supply sources and optimizing logistics to ensure a steady supply of raw materials.
Conclusion
The production of potassium bicarbonate is a multifaceted process that involves careful management of raw materials, energy, and labor. Understanding the production cost structure and staying abreast of the latest industry developments are crucial for manufacturers aiming to remain competitive in the market. As demand for potassium bicarbonate continues to grow, innovations in sustainable production methods and technological advancements will play a pivotal role in shaping the future of this essential compound. With a focus on efficiency and compliance with regulatory standards, the potassium bicarbonate industry is poised for significant growth and development in the coming years.